DEER PARK Ill June 15 2021 GLOBE NEWSWIRE -- Eton Pharmaceuticals Inc Nasdaq. In addition the company is also planning a third booster shot.
Moderna has asked the Food and Drug Administration for full approval of its coronavirus vaccine in people 18 and older.

Fda booster shot approval. Benjamin Burnett an analyst at Stifel expects the hold could delay Bluebird filing for approval in sickle cell until 2023. Plus the newest vaccine trial data how a lack of truck drivers could lead to a summer fuel shortage and the future of remote. On June 1 Moderna said that it had begun the rolling submission process to apply for full approval from the FDA for its COVID vaccine in those 18 and older.
On December 18 2020 the US. Moderna announced Wednesday its booster shot showed a positive immune response against the Covid-19. This shot will boost your immune response.
The company is the second vaccine maker to seek full approval from US. PfizerBioNTech has initiated its application to the US Food and Drug Administration for full FDA approval of its Covid-19 vaccine for people ages 16 and older the companies said Friday. Despite the success of the current COVID-19 vaccines drug makers are currently working on booster shots but medical experts say at least in the near term that may not be necessary.
What difference would full FDA approval make for COVID-19 vaccines. MODERNA SEEKS FULL APPROVAL OF COVID SHOT The company is seeking full FDA approval of its vaccine for use in those 18 and older it announced Tuesday morning. C OVID-19 vaccine maker Moderna has petitioned the Food and Drug Administration for full approval of its shots for use for adults 18 and older.
Food and Drug Administration issued an emergency use authorization EUA for the second vaccine for the prevention of. Information about the Moderna COVID-19 Vaccine. Last week the company applied to the US FDA for full approval for its mRNA Covid-19 vaccine for use in people 18 and older.
The company is seeking full FDA approval for use of its vaccine in adults over the age of 18. In addition the company is also planning a third booster shot. Moderna is the second Covid-19 vaccine maker to seek full approval following Pfizer and.
The manufacturer announced Wednesday that the booster shot it was testing was effective against. We are pleased to announce this important step in. Emergency use authorization allows a vaccine to become available prior to full approval in the case of public health emergencies.
ETON today announced that the US. The company is the second vaccine maker after Pfizer and its partner BioNTech to seek full FDA approval. Moderna has since expanded its research to test the vaccine in younger people.
Moderna said last month that its shot proved 100 percent effective in teens ages 12 to 17 enrolled in a late-stage clinical trial. The FDA will scrutinise the information to see if the vaccine meets stringent criteria for full licensure. Moderna said Thursday it would submit its Covid-19 vaccine for full FDA approval by the end of May.
The plan is for every adult to get a booster shot eight months after you got your second shot he said. In summary the FDA has not released specifics on size or timing for booster shot trials but with our sources we can verify clinical trial data is required and will study how well a booster shot. Currently no COVID-19 vaccine is fully approved by the FDA but three - Moderna Pfizer and the currently questionable Johnson Johnson - were given emergency use authorization by the agency.
William Schaffner MD medical director of the National Foundation for Infectious Diseases tells Verywell that the FDA full approval process is more rigorous compared to an EUAIt involves reviewing all of the data regarding the effectiveness of the vaccine its safety many aspects of its manufacturing process including inspections of the facilities where its manufactured and also. It will increase your protection from COVID-19. Bluebird can now move forward with completing an approval application to the FDA in beta-thalassemia submission of which had been contingent on successful resolution the agencys concerns related to the cancer cases.
Food and Drug Administration FDA has approved Rezipres ephedrine. The company has also requested a priority review which asks that the FDA review and take action on its application within six months rather than the 10 months that is allocated with standard review per CNN. Last week the company applied to the US FDA for full approval for its mRNA Covid-19 vaccine for use in people 18 and older.

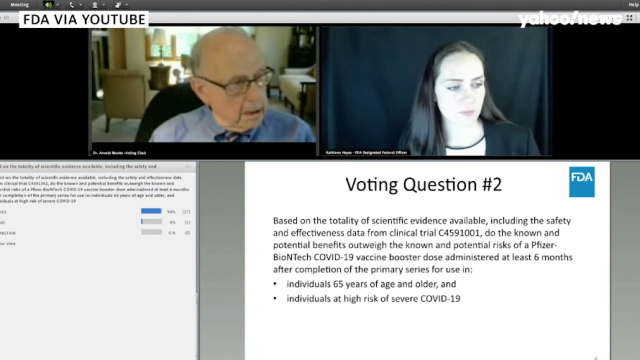
Fda Vaccine Advisory Committee Rejects Biden S Booster Shot Plan For General Population Health News Us News

Covid Vaccine 3rd Dose Limited To Immunocompromised As World Health Organization Calls For Booster Moratorium Abc7 Chicago
Moderna Begins Submission Process For Fda Approval Of Its Covid Booster Shot Barron S

Fda Recommends Covid Vaccine Booster Shots For Ages 65 And Up People Com

Fda Recommends Covid Vaccine Booster Shots For Ages 65 And Up People Com