To seek a biologics license that will allow it to market the shots directly to consumers. World Health Organization chief urges halt to booster shots for rest of the year By KEVIN McGILL and MELINDA DESLATTE.
11 new deaths in Louisiana reported from Ida raising states death toll to.
Fda booster meeting. Provide price capping waiver indemnity. Populations that might be targeted for booster doses include residents of long-term care. Earlier this month a group of twenty-seven scientists clinicians and patient advocates submitted a formal Citizen Petition with the United States Food and Drug Administration FDA requesting the agency to halt any consideration of a full approval of COVID19 vaccines.
The US Centers for Disease Control and Prevention and the US Food and Drug Administration are recommending that the United States pause the use of Johnson Johnsons Covid-19 vaccine over six. Information about the Moderna COVID-19 Vaccine. A Food and Drug Administration advisory committee will hold an all-day meeting Friday to review the data and is likely to give the vaccine a thumbs-up leading to an expected FDA.
BioVaxys submitted a Pre-IND meeting request and briefing package with the FDAs Center for Biologics Evaluation and Research CBER for CoviDTH in March of this year. June 24 2021 -- The US. Vaccines and Related Biological Products Advisory Committee meeting December 10 2020.
Human Rabies Prevention United States 1999. In general new drugs may not be introduced or delivered for introduction into interstate commerce without an approved application from FDA in effect as described in section 505a of the FDC Act 21 USC. Vaccines and Related Biological Products Advisory Committee meeting December 17 2020.
On Tap Moderna is seeking full FDA approval of its Covid-19 vaccine the second manufacturer to do so. A Adapted from the Recommendations of the Advisory Committee on Immunization Practices. Vaccines and Related Biological Products Advisory Committee Meeting December 10 2020 FDA Briefing Document Pfizer-BioNTech COVID-19 Vaccine Sponsor.
Cipla to Govt Ciplas latest communication to the govt dated May 29 follows a high-level meeting held recently during which it was discussed that Moderna has proposed to launch a single-dose vaccine for the Indian market for which. The message from the experts is slow down and get the science rightthere is no legitimate reason to. With Katherine Ellen Foley.
But a CDC advisory panel where the FDA made the announcement still strongly backs the vaccines benefits which outweigh the rare risk for heart inflammation. The mRNA vaccine is currently on the US. The paper said Englands Health Secretary sat on positive data for 3 days prior to meeting that ruled unlocking must.
The FDA is poised to authorize PfizerBioNTechs coronavirus vaccine in children and teens ages 12 to 15 by early next week a federal government official tells CNN. Food and Drug Administration issued an emergency use authorization EUA for the second vaccine for the prevention of. FDA briefing document Moderna COVID-19 vaccine.
355a unless they are nonprescription drugs governed by and lawfully marketed under section 505G of the FDC Act. FDA to Add Warning on Rare Myocarditis Risk After COVID Vaccination. Pfizer Comes Close To Meeting FDAs Guidance Standard For COVID Booster Dose But It May Not Be Enough 23 hours ago Real-World Evidence Will Take Center Stage At US FDA Advisory Committee On COVID Boosters 15 Sep 2021.
Moderna is the second drugmaker in the US. Close to commit over 1-bn to Moderna for Covid-19 booster vaccine. Food and Drug Administration.
PFE and Moderna Inc NASDAQ. The FDA on Friday added a warning to patient and provider fact sheets for the Pfizer Inc NYSE. Rabies Postexposure Prophylaxis Guide United States 1999 a.
1 b During the 10-day observation period begin postexposure prophylaxis at the first sign of rabies in a dog cat or ferret that has bitten someone. Autumn Booster Jab Planning. On December 18 2020 the US.
Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19. Market under an.


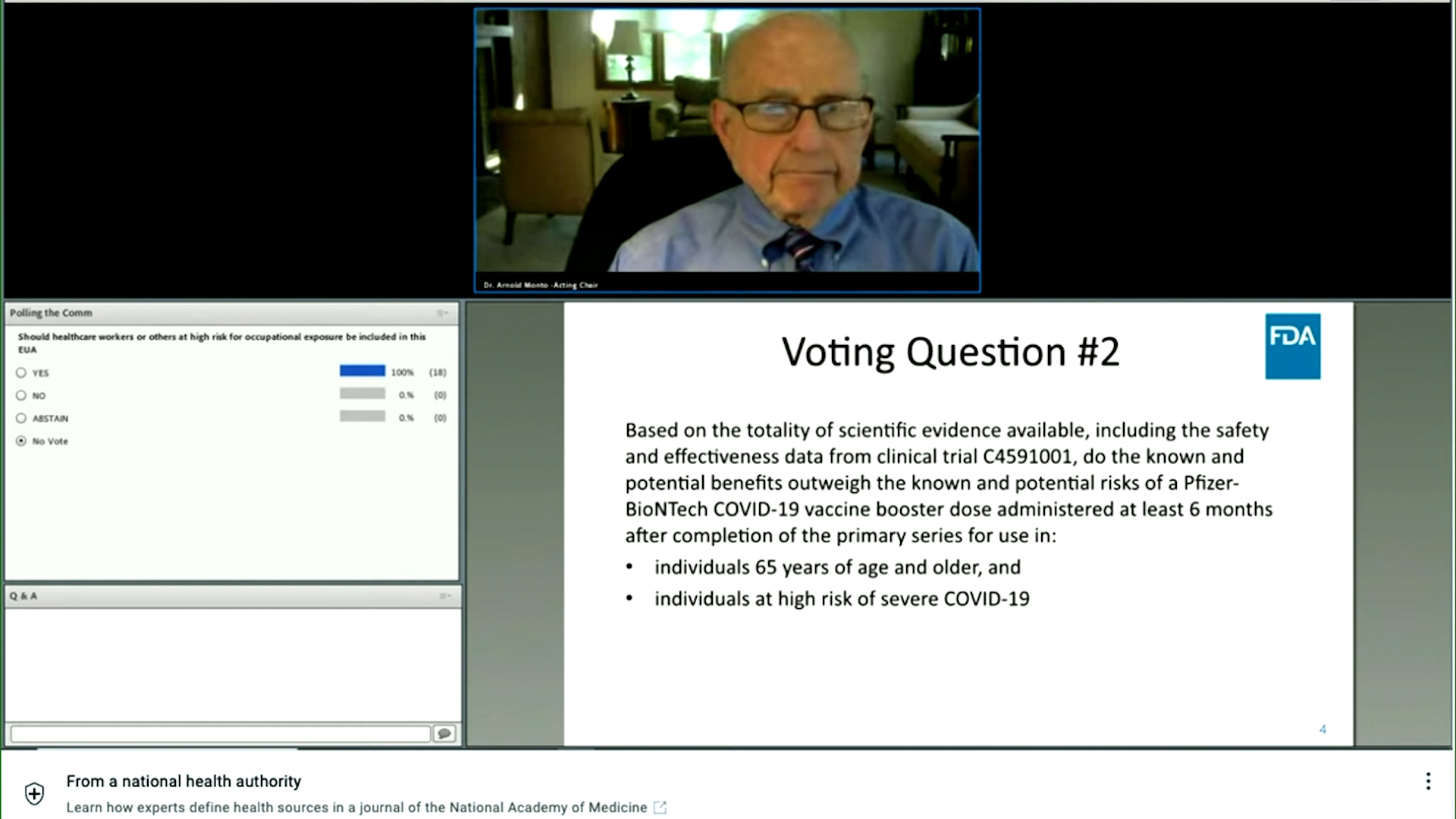






/do0bihdskp9dy.cloudfront.net/09-17-2021/t_a164a45c495b4310a064abdaafb676b4_name_file_1280x720_2000_v3_1_.jpg)

/do0bihdskp9dy.cloudfront.net/09-19-2021/t_62e70ed59e4c49b1a8697975f9a89456_name_file_1280x720_2000_v3_1_.jpg)



/do0bihdskp9dy.cloudfront.net/09-17-2021/t_008492888af84d07b3e1a53011290273_name_file_1280x720_2000_v3_1_.jpg)
